
🔥 Pro Tip: Remember your stoichiometric coefficients! The real equation has you multiplying by the stoichiometric coefficient of each product/reactant but the CollegeBoard omits it from the formula for some unknown reason. This can be applied the same way we did in unit 5 with enthalpy, so you can simply use the table and plug in numbers for each product/reactant.
/GettyImages-697595142-5babd90746e0fb0025f7cf17.jpg)
This equation tells us that for a reaction the overall entropy change is the sum of the changes in the entropies of the products minus the sum of the entropies in the reactants. Therefore, the distance traveled is not a state function and is not pathway independent. If you go straight up, the distance will differ from if you zigzag and for any other path you take. On the other hand, the distance you travel up the mountain does depend on the path you take. This makes this function pathway independent. Whether you went straight up, zigzagged, went curvily, or any other pathway, your change in altitude will be the same at the end. Since no matter which way you go, you will end up at the top of the mountain, your change in altitude will always be the altitude. An example of a state function is the change in altitude when climbing a mountain, whereas an example of a non-state function is the distance traveled. This means that whatever “path” you take to get to the end result, the end result will be exactly the same.
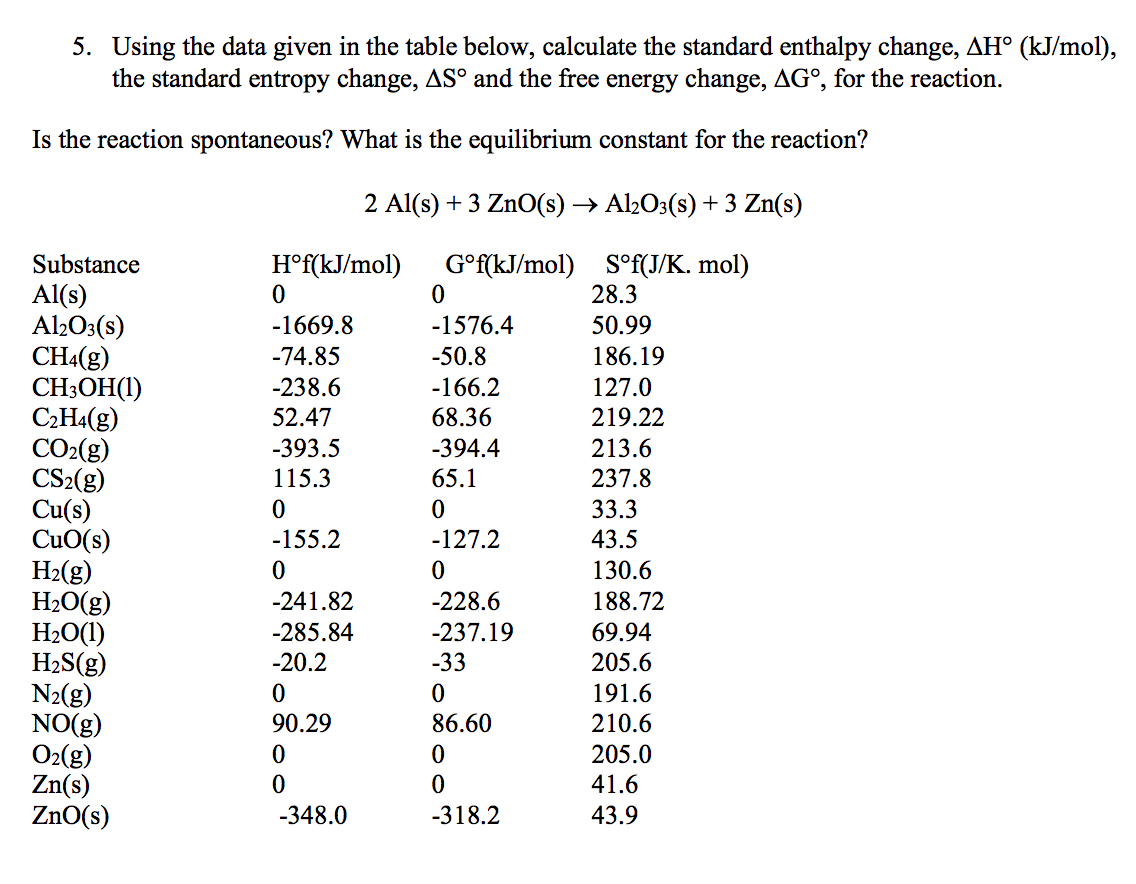
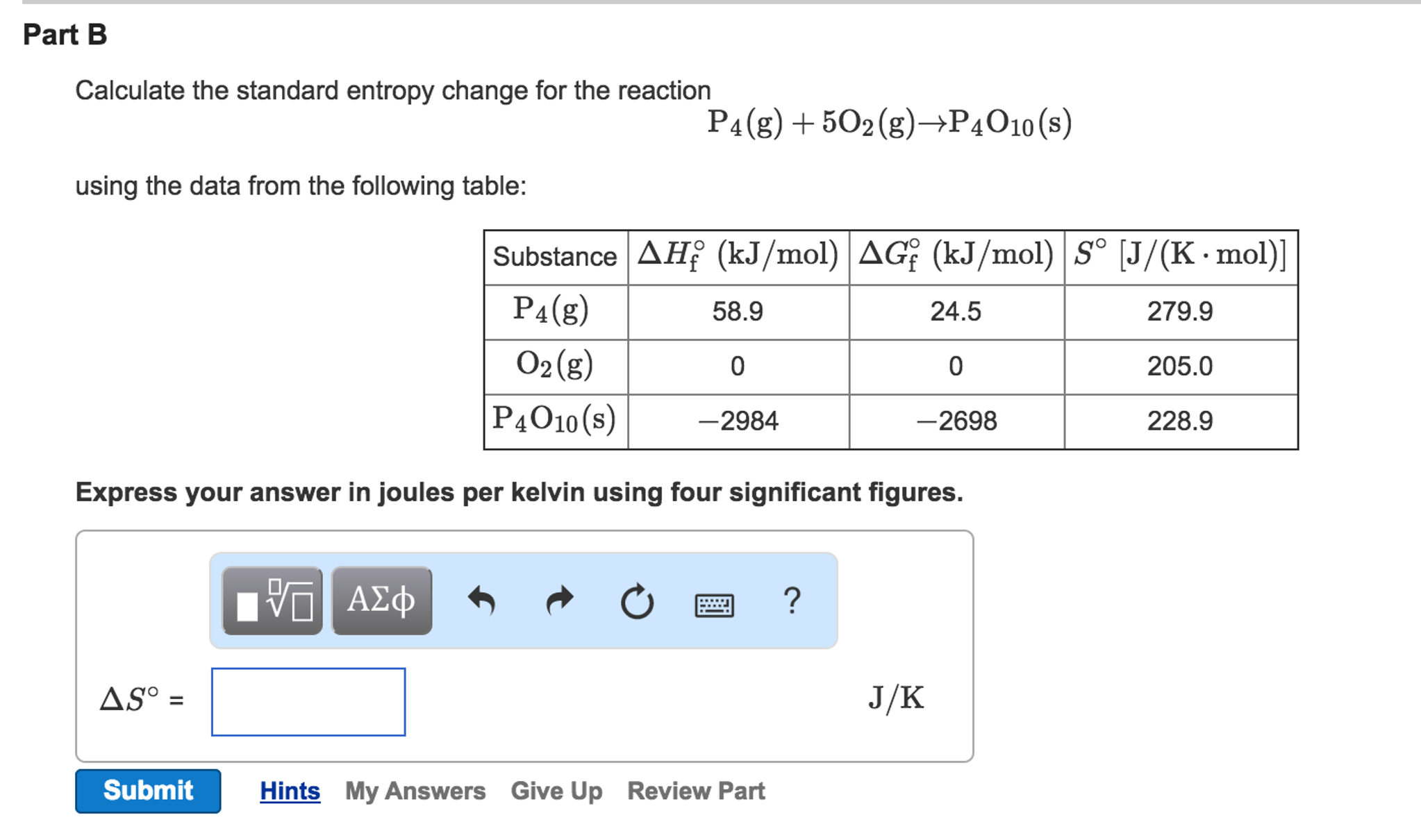
In essence, a state function is a function that has the property of pathway independence. Let’s take a quick side note to explain what a state function means and why it’s important. In Unit 6, we learned about Hess’s Law, which told us that enthalpy was a state function, meaning enthalpy changes are pathway independent. For now, lets look at the far right column that says S°.Ĭhange in entropy (ΔS°) can be thought of the same way we thought about changes in enthalpy (ΔH°) in unit 6 just thinking about entropy instead of heat changes. These tables may also contain other data such as enthalpies of formation like the one below. Similarly, most if not all chemistry textbooks have tables of thermodynamic data in the back of the book with a chart of standard entropies. Calculating these values is incredibly complex, but you will be given any absolute entropies you need on the exam. Absolute entropies, also known as standard entropies, describe the number of possible states a molecule can take, a measure of its disorder. Comparing S and ΔSĮntropy can be thought of both in its absolute form (S°) and as representing a change in entropy (ΔS°) (note that the ° symbol in each simply means that we are at standard conditions, that is 1atm of pressure and 273K). This differs from enthalpy, which can only be represented as a change (we can never find H° for a reaction but we can find ΔH°). Unlike enthalpy, which was discussed in unit 5, entropy can be measured both absolutely and as a change. In the last section, we introduced the idea of entropy as a measure of disorder in a system.


 0 kommentar(er)
0 kommentar(er)
